
RECOMMENDATIONS No 7
Webversion 12.02.1999
This document is the HTML-version of the published version ISBN
3-908125-23-5
To obtain the published version contact the corresponding author
Members of the working group
![]() Dr. Ernst BORN
Dr. Ernst BORN
Abt. für Med. Strahlenphysik, Universität Bern, Inselspital,
3010 Bern
![]() Antonella FOGLIATA-COZZI
Antonella FOGLIATA-COZZI
Ospedale San Giovanni, 6500 Bellinzona
![]() Florica IONESCU
Florica IONESCU
Rätisches Kantonsspital, Radio-Onkologie, 7000 Chur
![]() Dr. Victor IONESCU
Dr. Victor IONESCU
Hirschbühlweg 18, 7000 Chur
![]() Pierre-Alain TERCIER *,
Pierre-Alain TERCIER *,
Institut de
Radiophysique Appliquée, Centre universitaire, 1015
Lausanne
new address: Service de Radio-oncologie,
HFR - Hôpital Cantonal, 1708 Fribourg,
![]()
*Corresponding author
![]()
1. COMMISSIONING
1.1.
PREREQUISITES OF COMMISSIONING
1.1.1. Vendor
Tasks
1.1.2. User and System Administrator Tasks
1.1.3. Preliminary Checks for Commissioning
1.2. CHECKS
FOR COMMISSIONING
1.2.1. Non-Dosimetric and Non-Geometric Checks
1.2.2.
Geometry
1.2.3.
Devices (peripherals)
1.2.4. Dosimetric Measurements for TPS Validation
2. TESTS AFTER
IMPLEMENTATION OF A NEW SOFTWARE RELEASE
2.1. INTRODUCTION
2.2. ACCEPTANCE TESTS FOR A NEW SOFTWARE RELEASE NOT
AFFECTING PHYSICAL ALGORITHMS
2.2.1. Non
dosimetric checks
2.2.2.
Dosimetric checks
2.2.3. Verification of all potentialities of the TPS
3. TESTS AFTER ADDITION OF A NEW THERAPY UNIT AND/OR IMPLEMENTATION OF NEW BEAM DATA
4. REPEATED TPS CHECKS
4.1. INTRODUCTION
4.2. TESTS AND
FREQUENCIES
4.2.1. Peripherals
4.2.2.
Monitor Units (MU)
4.2.3.
Standard treatment plan
4.2.4.
"Checksum"
With the introduction of CT based computerised treatment planning in radio-oncology the treatment planning system (TPS) has become a key element in the radiotherapy process. Regarding patient safety and success of therapy, its accurate and stable functioning is an issue of highest importance. For these reasons quality assurance (QA) procedures have to be implemented for the commissioning phase and for the clinical routine running of a TPS.
Being a hardware and software system, each TPS may incorporate errors [14] from any of these different kinds:
This recommendation is the counterpart of the issue of the SSRMP "Physical and Dosimetric Checks in Teletherapy" [27]. Its purposes are defined in the same way:
It is the medical physicist's responsibility to implement the QA procedures described in this document
Chapter 1 describes QA Procedures (QAP) for commissioning a TPS for both photons and electrons beams, chapter 2 and 3 deal with QA aspects after implementation of new software releases and new beam data respectively and chapter 4 lists QA recommendations for routine use of a TPS. After a bibliography of related literature, the appendix contains a summary of tolerances and frequencies of tests.
Future publications of QA guidelines by other national or international authorities, may necessitate a revision of this document.
Commissioning of a TPS by a qualified medical physicist is necessary before the system is used for clinical purposes. Commissioning has to validate the proper functioning of the system according to the vendor's specifications and to clinical requirements. The results can be used to establish standards of acceptance for the demonstration of the correct working of the TPS in regular QA-checks. Calculations for commissioning test cases shall be compared against measurements done at the same time as those used to characterise the treatment unit model2.
1.1. Prerequisites of Commissioning
Prior to the commissioning process, the following points and suggestions should be considered.
TPS hardware tests are required in order to ensure that both the computer and its peripherals are operating according to specifications. Most computers have an automatic system diagnostics that include tests for the processor, memory and disk operation. In addition the following items are to be tested:
Allowed ranges and directions of linear and rotational scales for technical treatment machine parameters should correspond to the actual situation for individual treatment units. If not, this shall be taken into account and a specific procedure shall be defined concerning the transfer of set-up information from treatment plans to the corresponding therapy unit.
The data transfer from TPS to the simulator and/or the accelerator shall be tested.
All the parameters describing beams shall be checked for completeness, availability and consistency on screen or paper (e.g. isodose chart).
All patient identification details shall be checked for completeness, availability, consistency and uniqueness. All reproductions on hard copies or printed pages shall be complete with regard to date, time, plan identification number and version number of the calculation.
An independent check shall be undertaken to ensure that the basic input data has been entered correctly. This can be done by printing out tables of entered beam characterisation parameters, e.g. values of TAR, TPR, depth doses, output factors etc., if the algorithm is based on tabulated data. The aim is to ensure correctness of data input.
The accuracy and completeness of the 3D-models of the patient and the beams have to be checked (the required tolerance regarding to distances is 0.1 cm). Every TPS has it's own functions and these have to be tested according to their use. The following list shall be completed according to the TPS particularities:
Note : Not all of these options are available in all TPS.
On each treatment plan printed or plotted, two scales in both directions (horizontal and vertical) with centimetre marks at least over 10 centimetres are required. However all the marks shall be verified with a ruler, they must be at the right place (i.e. no distortion and no displacement).
The vertical and horizontal scales are to be tested along 10 cm showing an agreement better than 0.1cm.
An object on a film with particular dimensions is scanned. The size displayed by the TPS is then compared to the real one.
General QAP of CT are not the subject of these recommendations, additional information on CT QAP can be found in the literature [19],[20],[25],[32]. Issues covered here are the transfer of CT data to the TPS and the use of CT data by dose calculation algorithms.
Geometrical distorsions shall be less than 0.1 cm
To give and idea of the expected accuracy :
Information to produce blocks (and compensators) have to be transferred from the TPS to the block cutting device. The proper functioning of data transfer shall be checked by examining blocks of clinically relevant shapes.
If the TPS includes an archiving system, the correctness of treatment plan parameters and patient data read back from the archive has to be checked (correct treatment plans irrespective of TPS development and addition of therapy units etc.). The scale of outlines, the shape of the beam, the date (for a Cobalt-60 unit) are to be checked.
The dosimetric measurements for TPS validation have two goals; test the accuracy of point dose calculation and interpolation (or analytic computation) procedures. It is good practice to use for the acquisition of control data sets a better spatial resolution than those imposed by the TPS for the acquisition of the basic reference data. The same consideration should be made for the choice of the measuring depths.
Tolerance limits for the calculation of relative dose distribution in region of low dose gradient, are quoted as percentage of the central axis dose in a single field case or as percentage of the dose at the prescription point in a clinical case. In areas of high dose gradient (penumbra, build-up) the tolerance should also be specified in terms of displacement (shift of isodose in cm) of corresponding dose values. The level of acceptance stated in this report is based on the experience of different authors and reflects the precision actually achievable [8],[15],[11],[18],[22]. For each recommended measurement a particular tolerance is given. Deviations in some regions or under some circumstances in excess of the tolerance limits have to be judged in the light of their clinical relevance.
The part concerning the calculation of Monitor Units (MU) is reported in the section concerning the repeated checks. You should also refer to the section 4.2.2 for the commissioning.
The following tests shall be made for each photon beam quality and each therapy unit available.
(i) Beam characterisation data
The aim of this test is to check the basic beam data which is used by the planning system to set up a beam model. Algorithms in commercially available planning systems are based either on tabulated beam data (A) or on analytical treatment unit models (B). Checking beam characterisation data for case (A) means direct checking of the tabulated data by suitable means (graphs, tables, software tools). Errors are not expected and not accepted in these checks. In case (B), beam characterisation data is used to fit some more fundamental model parameters. Some deviations therefore may be expected when beam characterisation situations are recalculated and the calculated values are not necessarily identical to the characterisation data. The following table lists dose differences and isodose shift distances between calculated and measured results, which are considered to be acceptable.
| Testing set |
| The data used for beam characterisation (TMR, PDD, TAR, TPR, profiles, output factor and relative output factor) shall be checked by comparison with recalculation. The cases (A) and (B) have different tolerances. In the region of high dose gradient, the distance between isodose lines is more appropriate than % difference. |
| Description | Différence in % of the maximum | Shift of isodoses [cm] |
| TMR, TAR, TPR or PDD on beam axis, profiles | A : no diff. B : 1 |
- B : 0.1 |
| Description | % of the measured factor |
| Dose per Monitor Unit6 (for the reference field e.g. 10x10 cm2) | A : no diff. B : 0.5 |
| Relative output factor7 | A : no diff. B : 1 |
(ii) Open and wedged beams for fields not used for beam characterisation (field sizes of clinical relevance, either interpolated or outside characterisation range)
All these tests are to be performed for the proposed minimal set of fields (assuming that these fields were not used for beam characterisation).
| Testing set |
| Description These tests shall be performed by means of calculation of point doses and/or dose distributions compared with measurements. Minimal set of field sizes: - 5x5, 10x10, 25x25, 40x40 (max. square field) - 5x10, 5x25, 5x40, 10x5, 25x5, 40x5 |
Difference in % of the maximum | Geometric differences [cm] |
| PDD on beam axis (except in the build-up region) | 2 | |
| PDD in the build-up region | 0.2 | |
The dose profiles (or isodoses) in main (X and Y)
and diagonal directions and at 5, 10, 20 cm
depth:
| 2 | |
The dose profiles (or isodoses) in main (X and Y)
and diagonal directions and at 5, 10, 20 cm
depth:
|
0.2 | |
| Depth dose at a point along the beam central axis for different SSDs clinically used (e.g. 80 and 120 cm) at different depths (e.g. 5, 10 and 20 cm) and square fields). | 2 |
| Description | Difference (% of the measured value) |
| Relative output factor | 2 |
(iii) Irregular, multileaf collimated, asymmetric and dynamically wedged fields
Although all these features may be present in one single field in clinical practice, separated tests should be performed for each of them.
| Description of the testing set |
| Description These tests shall be performed by means of calculation of point doses and/or dose distributions compared with measurements. |
Diff. in % of the maximum | Geometric differences [cm] |
| PDD in an open part of the field (not in a penumbra region) | 2 | |
| PDD in the build-up region | 0.2 | |
Dose profiles (in any direction) and at 5, 10, 20
cm depth:
|
3 | |
Dose profiles (in any direction) and at 5, 10, 20
cm depth:
|
0.3 | |
| Dose behind large blocks (or multileaf
collimator) (it measures the accuracy of
calculated transmission) Note: Doses under small blocks are difficult to calculate and to measure, the precision should be estimated for situations corresponding to clinical applications |
2 |
| Description | Difference (% of the measured value) |
| Relative output factor | 2 |
The output of the reference field for different irradiation times shall be tested.
| Testing set |
| Description This test shall be performed by means of TPS calculations compared with hand calculations. |
max. difference [%] |
| Output of reference field and for reference conditions (dose per unit of time) | 0.5 |
(v) Inhomogeneity correction, surface irregularities and oblique incidence fields
The dose per monitor unit on the central ray in region of electronic equilibrium shall be tested. Some cases of inhomogeneities are proposed and described in the figure below. Of course, other situations of clinical relevance can also be verified. As the measurements can be difficult [28], it is a good practice to compared with situations found in the literature [24].
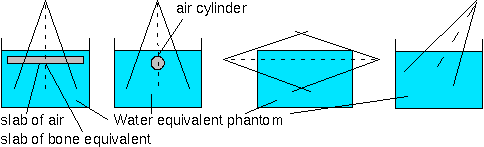
figure 1. Description of a proposed testing set
| Description of the testing set |
| Description These tests shall be performed by means of calculations compared to measurements. |
% of the measured factor |
| Dose per monitor unit | 3 |
(vi) Standard treatment plans (3D distributions, rotational irradiation, multiple beams)
Several anthropomorphic phantom tests can be used for a final complete test of the entire calculation algorithm. These test cases should be similar to treatment techniques used in the clinic, and of interest to the physicians and physicists involved. Some examples:
| Description of the testing set |
| Description These tests shall be performed by means of calculations compared to measurements. |
% of the normalisation dose |
At the point of reference (ICRU point as defined in
ICRU Report 50 [12]) and anywhere in
low dose gradient region
|
4 |
For electrons, the dose gradient is steep along the central axis beyond the maximum and at beam edges. In order to avoid uncertainty in the position of the measurement high resolution detectors (diode detector, film, small ionisation chambers) and detectors which have a well defined effective points of measurement should be used (plan parallel ionisation chamber). In the conversion from depth ionisation (ionisation current) to depth dose the SSRMP protocol should be used.
The contents of the test shall include planar dose distributions in a number of planes: (a) a transverse plane containing the central axis of the beam, (b) a transverse plane a few centimetres off axis, (c) planes normal to the beam axis (beam's-eye-view planes) at several depths [33]. Normal plane isodoses verify the fluence distribution over the entire surface which in combination with the depth doses enables beam data verification over the whole volume.
As stated by many authors, the difference between measured and calculated dose could be relatively large (10%) [8],[9],[16] in the case of electron beams. The following tolerances should be regarded as ideal.
The following tests shall be made for each electron beam quality and each therapy unit available.
(i) Beam characterisation data
The aim of this test is to check the basic beam data which is used by the TPS to set up a beam model. Algorithms in commercially available planning systems are based either on tabulated beam data (A) or on analytical treatment unit models (B). Checking beam characterisation data for case (A) means direct checking of the tabulated data by suitable means (graphs, tables, software tools). Errors are not expected and not accepted in these checks. In case (B), beam characterisation data is used to fit some more fundamental model parameters. Some deviations therefore have to be expected when beam characterisation situations are recalculated and the calculated values are not necessarily identical to the input data.
| Testing set |
| Description The data used for beam characterisation (PDD, profiles, output factors and relative output factors) shall be checked by comparison with recalculation. The cases (A) and (B) have different tolerances. |
Différence in % of the maximum | Shift of isodoses [cm] |
| PDD on beam axis or profiles | A : no diff. B : 1 |
- B : 0.1 |
| Description | % of the measured factor |
| Dose per Monitor Unit (for the reference field) | A : no diff. B : 0.5 |
| Relative output factor | A : no diff. B : 1 |
(ii) Open fields not used for beam characterisation (field sizes of clinical relevance, either interpolated or outside the characterisation range)
All these tests shall be performed for a minimal set of field sizes. The field sizes to be included in this set are electron energy and accelerator specific. They could depend also on the calculation algorithm and shall be chosen in order to get an idea where possible weaknesses in the dose calculation would occur. For accelerators using electrons applicators, tests for all of them shall be performed.
| Testing set |
| Description These tests shall be based on a comparison of the computer calculated dose distributions with measured electron beam dose distribution. As a minimal test, dose profiles at two different depths should be measured to verify both underlying angular and fluence distribution of the electrons. It is recommended to consider profiles at the depth of the clinically relevant isodose outlines (say, 100%, 90%, 80%, 50%). |
Diff. in % of the maximum | shift of isodose [cm] |
| PDD on beam axis within the therapeutic range (up to 80% dose level) | 1 | |
| PDD on beam axis beyond the therapeutic range | 2 | 0.2 |
| Practical range Rp | 0.2 | |
The dose profiles (or isodoses) in main directions (X
and Y and diagonal)
|
2 | |
The dose profiles (or isodoses) in main directions (X
and Y and diagonal)
|
0.2 |
| Description | % of the measured value |
| Relative output factor | 2 |
| Dose per Monitor Unit (MU) at extended and reduced SSD encountered in the clinical situations. | 2 |
(iii) Irregular field shapes, asymmetric and oblique incidence
Situations that would be encountered in the clinical treatment planning should be evaluated: oblique incidence as in the irradiation of the neck or chest wall, irregular geometry as the irradiation of tumours in and behind the nose, fields with low melting point alloy blocking, dose distribution behind bolus.
| Description of the testing set |
| Description These tests should be based on a comparison of the computer calculated distribution with the experimentally obtained electron beam distribution. |
Diff. in % of the maximum | Shift of isodoses [cm] |
| PDD on beam axis | 2 | 0.2 |
The dose profiles (or isodoses) in main directions (X
and Y) and at minimum two depths (see. Section c
(ii)):
|
3 | |
The dose profiles (or isodoses) in main directions (X
and Y) and at minimum two depths (see. Section c
(ii)):
|
0.3 |
| Description | % of the measured factor |
| Relative output factor | 3 |
(iv) Inhomogeneity correction and irregularities of the surface
The dose per monitor unit below slab inhomogeneity of lung and bone density in point of electronic equilibrium shall be tested.
| Description of the testing set |
| Description These tests shall be performed by means of calculation compared to measurements. |
% of the measured factor |
| Dose per monitor unit | 5 |
Several anthropomorphic phantom tests can be used for a final complete test of the entire calculation algorithm. These test cases should be similar to treatment techniques used in the clinic, and of interest to the physicians and physicists involved (the nose, mandible, etc.). Particular attention shall be paid to the superposition and matching of field, (also with mixed photon and electron beams according to the TPS capabilities).
| Description of the testing set |
| Description These tests shall be performed by means of calculations compared to measurements. |
% of the normalisation dose |
At the point of reference (ICRU point as defined in
ICRU Report 50 [12]) and anywhere in
low dose gradient region
|
5 |
Tests after implementation of a new software release
After any software upgrade (installation of new releases or other modification) it is recommended to check the reliability, correctness and constancy of relevant parameters and of calculation algorithm. To be more efficient, a detailed study of the changes expected in the new version, and good documentation of all the expected changes shall be furnished by the vendor [34]. New software releases and upgrades could be classified into two subsets: those not directly influencing the calculation algorithm and those where substantial variation on physical models implemented are introduced (e.g. change in irregular fields treatment, head scatter corrections...)
In this latter case, given the complexity of TPS program structures, the whole calculation algorithm has to be considered changed and as a consequence all involved treatment units shall be checked as already described for commissioning.
In the case of software releases related only to ancillary or to presentation utilities (like graphic tools added or modified), only a subset of the commissioning procedure check are mandatory before the use of the TPS in clinical routine. The following paragraphs are devoted to this case suggesting an explicit verification list.
All clinically used tools and programs, whether or not involved in the software upgrade, shall be checked: coarse checks, geometry and 3-D functionality, peripherals (see paragraphs 1.2.1 to 1.2.3) need a control as for the commissioning (see paragraph 1.2). It is also important to check for the completeness of the functions, as this may interfere with clinical routine.
point dose at 3 depths (dmax, 5 cm, 10 cm) for 5x5 cm2, 10x10 cm2 and maximum open square fields, a rectangular 5x30 cm2 field and an irregular field.
profiles: for open beams (10x10 cm2 and the maximum square field) at 10 cm depth; for wedged beams only the maximum field allowed at 10 cm depth.
point dose for reference field, at 2 depths: dmax and a point in the fall off region
profiles in the same conditions
The dose distribution in at least the central beam section and one off-axis section shall be calculated. As recommended in section 1.1.3 (Preliminary check for commissioning), the vendor shall provide a standard and common set of patients and beam data with their output (dose distribution). This set shall be used to test the local implementation of the new software release allowing for inter-comparisons with other TPS. The results obtained from these test patients may be compared with the data obtained from the previous release by means of a systematic dose verification in predefined points and on isodoses distributions.
As an alternative method 3 real patients locally treated could be used to check the new version following the same procedure.
Isodoses, monitor units calculations and DVH results (when available) from the new release, compared with those obtained from the previous one shall match without any quantitative difference unless mentioned as corrections by the vendor.
The standard procedure defined in 1.1.2 shall be tested. This systematic method is intended to reduce necessary time to check all functions, menus and submenus and to avoid doubled checks.
If any task in any TPS related program does not work as expected, it shall be noted in the TPS log-book and precisely reported to the manufacturer customer support teams. The operators shall be informed and temporary alternative procedures set up.
Tests after addition of a new therapy unit and/or implementation of new beam data
The implementation of a new therapy unit or new beam data concerns all steps of a TPS Quality Assurance Program; this implies that in order to exclude any systematic error in treatment planning, all the functions relied to this new treatment unit have to be carefully checked and evaluated before clinical use. From this statement, it follows that a complete set of tests of the software and dose calculation is required as for commissioning. Recommendations described in paragraph 1.2.1 and 1.2.4 apply here.
The aim is to ensure the constancy of calculation, dose distribution and all outputs from the TPS. Errors can occur from 4 different parts of the whole process of treatment planning : (1) programs, (2) beam data, (3) peripheral devices or (4) operators. Regarding to QA, all these parts shall be periodically tested. The periodic tests shall be made by different operators of the TPS. The idea is that operators can have their own habits in the schedule of executions and consequently use different algorithms in the program and perhaps obtain divergent results. The results could be operator dependent, hence the reason to impose standard procedure for the use of TPS.
Repeated checks as formulated here are an important part of the QAP, but a continued vigilance on the part of the operator is also required. The idea is to recognise the more subtle problems or differences which may occur. Investigation may uncover important issues which shall be resolved [34].
"In vivo" dosimetry is an overall test of the TPS, this subject is left to the judgement of the physicist as it lies outside the scope of these guidelines.
In this section, the tests are to be performed as described for the commissioning (section 1.2). The frequency of the checks are abbreviated as follow:
| Frequency | Abbreviation |
| monthly | m |
| quarterly | 3m |
| yearly | y |
You should report to the paragraph mentioned to see more details.
| Description of the testing set |
| Description | Frequency | Tolerance |
| Printing/Plotting device (see section 1.2.3.a) | m | 0.1 [cm] |
| Digitizer (see section 1.2.3.b) | m | 0.1 [cm] |
| Film scanner (see section 1.2.3.c) | m | 0.1 [cm] |
Computer Tomography (see section
1.2.3.d)
|
m | 0.1 [cm] |
Computer Tomography (see section
1.2.3.d)
|
y and at each revision of the CT |
if rhoe- <=1.5 then 0.05 if rhoe- > 1.5 then 0.1 |
| Block cutting device (see section 1.2.3.e) | y | no diff |
| Archiving and reading back of patient data (see section 1.2.3.f) | y | no diff |
A test concerning the calculation of MU shall be performed monthly on clinical cases. See also the reference [6] for more details. The verification of the monitor unit calculation for each patient is not treated in this document, as it is not a part of the TPS QA.
| Description of the testing set |
| Description These tests shall be performed by means of calculation of the TPS compared to previous calculation. |
Tolerance |
| MU Photons beams | no diff. |
| MU Electrons beams | no diff. |
The tolerance given in paragraphs 1.2.4.b.vi and 1.2.4.c.v are relative to the measured dose at the point of normalisation in an anthropomorphic phantom. Here the tolerance (1%) concerns the reliability of the checks done during commissioning. At each point verified the divergence in dose should not exceed 1% of the dose at the point of normalisation.
| Description of the testing set |
| Description Standard patients/phantom (compare with previous tests, see section 1.2.4.b.vi and 1.2.4.c.v) |
Frequency | Tolerance |
| with the possibility of performing checksums on beam data and executable files | y | 1% |
| without checksums | 3m | 1% |
The program cannot be modified by the operators therefore it should be verified that it has not been corrupted by any virus or wrong operations. The beam data can be modified (e.g. new measurement more precise). Such modification of the beam data shall be check by the appropriate procedure as describe below. When the beam data are supposed to be definitive, the integrity of the executable files or beam data shall be certified. If a virus corrupts programs or somebody makes a wrong operation on the system, a checksum utility program pointing on the executables, beam data and other important files can identify the problem [4]. It is the task of the vendor to implement it. This recommended test cannot be avoided if periodic tests are performed with standard clinical cases. Checks fulfilled on standard clinical cases only reveal problems without any explanation. On the other hand checksum tests can identify the problem and they can be executed automatically at each start of the TPS (boot procedure). A good practice is to ensure that the checksum is carried out after a registered modification of a parameter.
| Description of the testing set |
| Description | Frequency | Tolerance |
| Executable and beam data files: This concerns the scripts, binary executables and beam data files. The checksum program shall compare the current executables files with a situation of reference done at the commissioning of the TPS and periodically updated. It shall also highlight deleted and new executable files. |
m | no diff |
[1] AAPM Report No. 55, Radiation Treatment Planning Dosimetry Verification, from the Task Group #23 of Radiation Therapy Committee, Published by the American Institute of Physics, New-York, 1995, ISBN 1-56396-534-8
[2] ACR/NEMA Standards Publication No 300-1988, ACR/NEMA 2.0
[3] British Journal Of Radiology, Supplement 25, Central Axis Depth Dose Data for Use in Radiotheraphy , Published by the British Institute of Radiology, 1996, ISBN 0 905749 38 3
[4] B. Curran, G. Starkschall, A Program For Quality Assurance of Dose Planning Computers, ACMP Symposium "Quality Assurance in Radiotherapy Physics", Galveston, TX, May 1991
[5] Dahlin H. Lamm IL. Landberg T. Levernes S. Ulso N., User requirements on CT-based computed dose planning systems in radiation therapy, Acta Radiologica - Oncology. 22(5):397-415, 1983.
[6] R.A. Dahl, E.C. McCullough, D.E. Mellenberg, A quality assurance program for monitor unit calculators, Med. Phys. 17 (1), 1990, 103-105
[7] ACR/NEMA Standards Publication PS3, DICOM3
[8] J. Van Dyk, R.B. Barnett, J.E. Cygler, P.C. Shragge, Commissioning and Quality assurance of treatment planning computers, Int. J. Rad. Onc. Biol. Phys. 26, 1993, 261-273
[9] K. Friedrich, M. Fippel, « Externe Dosisberechnung mit TMS-Planungsdaten, Z. Med. Phys. 6, 163-168, 1996
[10] ICRU Report 35, Radiation Dosimetry: Electron Beams with Energies Between 1 and 50 MeV, International Commission on Radiation Units and Measurements, Maryland, USA, 1984
[11] ICRU Report 42, Use of Computers in External Beam Radiotherapy Procedures with High-Energy Photons and Electrons, International Commission on Radiation Units and Measurements, Maryland, USA, 1987
[12] ICRU Report 50, Prescribing, Recording, and Reporting Photon Beam Therapy, International Commission on Radiation Units and Measurements, Maryland, USA, 1993
[13] IEC 62083, "Equipment for radiotherapy, nuclear medicine and radiation dosimetry", specially Ed. 1: "Electromedical equipment - Particular requirements for the safety of radiotherapy treatment planning systems", by the International Electrotechnical Commission, 30.10.1998 version
[14] J. Jacky, C. P. White, Testing a 3D radiation therapy program, Int. J. Rad. Onc. Biol. Phys. 18, 1990, 253-261
[15] T. Knöös, C. Ceberg, L. Weber, P. Nilsson, The dosimetric verification of a pencil beam based treatment planning system, Phys. Med. Biol. 39, 1994, 1609-1628
[16] A. Kosunen, H. Järvinen, S. Vatnitskij et al., Intercomparison of radiotherapy treatment planning systems for external photon and electron beam dose calculations, Radiotherapy and Oncology 29, 327-335, 1993
[17] G.J. Kutcher, L. Coia, M. Gillin, W.F. Hanson, Comprehensive QA for radiation oncology : Report of AAPM Radiation Therapy Committee Task Group 40, Med. Phys. 21 (4), 1994, 581-618
[18] McCullough EC. Krueger AM., Performance evaluation of computerized treatment planning systems for radiotherapy: external photon beams, Int.J.Rad. Oncol. Biol. Phys 6(11), 1980, 1599-1605
[19] P.C. Shrimpton, D.G. Jones, M.C. Hillier, B.F. Wall, J.C. Le Heron, K. Faulkner, NRPB Report R248, Survey of CT Practice in the UK, Part 1, by the National Radiological Protection Board, Oxon, UK, 1991
[20] P.C. Shrimpton, D.G. Jones, M.C. Hillier, B.F. Wall, J.C. Le Heron, K. Faulkner, NRPB Report R249, Survey of CT Practice in the UK, Part 2, by the National Radiological Protection Board, Oxon, UK, 1991
[21] Three-dimensional photon treatment planning report of the collaborative working group on the evaluation of treatment planning for external photon beam radiotherapy, Int. J. Rad. Onc. Biol. Phys. 21 1, 1991
[22] IPEMB Report 68, «A guide to commissioning and quality control of treatment planning systems», 1996, by the Institution of Physics and Engineering in Medicine and Biology, York, England, ISBN 0904 181 839
[23] U. Rosenow, Qualitätskontrolle in der Bestrahlungsplannung, Z. Med. Phys 1 (1991) 59-67
[24] C. Kappas, J.C. Rosenwald, Quality control of inhomogeneity correction algorithms used in treatment planning systems, Int. J. Rad. Onc. Biol. Phys. 32 3 (1995), 847-58
[25] Evaluation des performances et contrôle de qualité des scanneurs", juin 1990
[26] D.L. McShan, Comments on: Commissioning and Quality assurance of treatment planning computers, Int. J. Rad. Onc. Biol. Phys. 26, 1993, 371-372
[27] Recommandations No 1 : «Contrôles physiques et dosimétriques en téléradiothérapie», Société Suisse de Radiobiologie et de Physique Médicale (1982, rév. 1992)
[28] R.L. Stern, B. A. Fraass, A. Gerhardsson, D. L. McShan, K.L. Lam, Generation and use of measurement-based 3-D dose distributions for 3-D dose calculation verification, Med. Phys. 19 (1), 1992,165-173
[29] G.K. Svensson, Quality assurance in radiation therapy: physics efforts, Int. J. Rad. Onc. Biol. Phys. V10, Sup 1, 1984, 23-29
[30] H. Svensson, Quality assurance in radiation therapy: physical aspects, Int. J. Rad. Onc. Biol. Phys. V10, Sup 1, 1984, 59-65
[31] C.Westermann CF. Mijnheer BJ. van Kleffens HJ,Determination of the accuracy of different computer planning systems for treatment with external photon beams, Radiotherapy & Oncology. 1(4):339-47, 1984 Mar
[32] D.R. White and R.D. Speller - "The measurement of effective photon energy and linearity in computerized tomography" Brit. J. Radiol. 53, 1980, 5-11
[33] Shiu AS, Tung S, Hogstrom KR, Verification data for electron beam dose algorithms, Med. Phys.19,623-636 (1992)
[34] Teletherapy: Present and Future, Proceedings of the 1996 summer school, «Quality assurance for 3D treatment planning», p. 253-318, AAPM, ISBN: 1-888340-03-7
The two following tables represent a summary of all the tests that should be performed for the commissioning, respectively repeated QA procedures.
Commissioning checks (see chapter 1)
| Description | Tolerance | Extension | |
| 1.2.1 | Non-Dosimetric and Non-Geometric Checks | ||
| Definition of technical treatment machine parameters |
no diff. |
(1) | |
| Data transfer between TPS and simulator/treatment machine |
no diff. |
||
| Beam description |
no diff. |
||
| Patient identification for each plan/version |
no diff. |
||
| Introduction of data |
no diff. |
(2) | |
| 1.2.2a | Geometry and 3D functionality - Geometry | ||
| Transfer of patient data and images |
no diff. |
||
| Definition of VOIs |
0.1 cm |
||
| Outline of the shaped beam in a section |
0.1 cm |
||
| Bolus (modification of patient's outline, associated with beams or not, density inside) |
0.1 cm + verif. |
||
| 1.2.2.b | Geometry and 3D functionality - 3D options | ||
| Mesh of 3D surfaces | |||
| - extreme cases |
verif. |
||
| - geometrically simple objects |
0.1 cm |
||
| - interpolated outlines |
verif. |
||
| Extracted outline | |||
| - general limitations |
verif. |
||
| - multiples outlines/section |
verif. |
||
| - shape of geometrically simple objects in section |
0.1 cm |
||
| 1.2.2.c | Geometry and 3D functionality - Beam's-eye-view | ||
| Shape and position of geometrically simple objects |
0.1 cm |
||
| Shape and position of the beam |
0.1 cm |
||
| DRR |
0.1 cm |
||
| 1.2.2.d | Geometry and 3D functionality - Dose Volume Histogram | ||
| Total volume |
verif. |
||
| Consistency checks with the isodoses charts |
verif. |
||
| 1.2.3 a | Devices - Printing/Plotting device | 0.1 cm | |
| 1.2.3 b | Devices - Digitizer | 0.1 cm | |
| 1.2.3 c | Devices - Film Scanner | 0.1 cm | |
| 1.2.3 d | Devices - CT | ||
| CT shape of particular object |
0.1 cm |
||
| Electronic density (rhoe- ) in function of CT#, (rhoe- ) compared to known densities relative to water |
0.05 / 0.1 |
(4) | |
| 1.2.3 e | Devices - Block cutting device | no diff. | |
| 1.2.3 f | Devices - Archiving and reading back of patient data | no diff | |
| 1.2.4 b | Photon dosimetry | ||
| 1.2.4 b (i) | Beam characterisation data | (3) | |
| TMR,TAR,TPR,PDD on beam axis and profiles | A : no diff. B : 1% or 0.1 cm |
||
| Dose per Monitor Unit for the reference field | A : no diff. B : 0.5% |
||
| Relative output factor | A : no diff. B : 1% |
||
| 1.2.4 b (ii) | Open and wedged beams | (5) | |
| PDD on beam axis excepted build-up region |
2% |
||
| PDD in the build-up region |
0.2 cm |
||
| The dose profiles (5,10,20 cm depth) | |||
| - high dose, low dose gradient |
2% |
||
| - low dose, low dose gradient |
2% |
||
| - high dose gradient (>30%/cm) |
0.2 cm |
||
| - the beam size defined by the 50% isodose |
0.2 cm |
||
| - the penumbra defined by the 20%-80% distance |
0.2 cm |
||
| SSD correction |
2% |
||
| Relative output factor |
2% |
||
| 1.2.4 b (iii) | Irregular, MLC, multileaf collimated, asymmetric and dynamically wedged fields | ||
| PDD in an open part of the field |
2% |
||
| PDD in the build-up region |
0.2 cm |
||
| The dose profiles (5,10,20 cm depth) | |||
| - high dose, low dose gradient |
3% |
||
| - low dose, low dose gradient |
3% |
||
| - high dose gradient (>30%/cm) |
0.3cm |
||
| Dose behind large blocks |
2% |
||
| Relative output factor |
2% |
||
| 1.2.4 b (iv) | Co-decay, output of the reference field and conditions using different irradiation times | 0.5% | |
| 1.2.4 b (v) | Inhomogeneity correction, surface irregularities and oblique incidence fields | 3% | |
| 1.2.4 b (vi) | Standard treatment plans | 4% | |
| 1.2.4 c | Electron dosimetry | ||
| 1.2.4 c (i) | Beam characterisation data | (3) | |
| PDD on beam axis or profiles | A : no diff. B : 1% or 0.1cm |
||
| Dose per Monitor Unit for the reference field | A : no diff. B : 0.5% |
||
| Relative output factor | A : no diff. B : 1% |
||
| 1.2.4 c (ii) | Open fields not used for beam characterisation | ||
| PDD on beam axis | |||
| - within the therapeutic range |
1% |
||
| - beyond the therapeutic range |
2% or 0.2 cm |
||
| - another suitable parameter Rp |
0.2cm |
||
| Profiles (depth of 100,90,80,50%) | |||
| - low dose gradient |
2% |
||
| - high dose gradient |
0.2cm |
||
| Beam size |
0.2cm |
||
| Relative output factor |
2% |
||
| Dose per Monitor Unit at extended and reduced SSD |
2% |
||
| 1.2.4.c (iii) | Irregular shaped field, asymmetric, oblique incidence field | ||
| PDD on beam axis | |||
| - low dose gradient |
2% |
||
| - high dose gradient |
0.2cm |
||
| Profiles (depth of 100,90,80,50%) | |||
| - low dose gradient in high dose region |
3% |
||
| - low dose gradient in low dose region |
3% |
||
| - high dose gradient (>30%/cm) |
0.3cm |
||
| Beam size defined by the 50% isodose |
0.3cm |
||
| Relative output factor |
3% |
||
| 1.2.4.c (iv) | Inhomogeneity correction | 5% | |
| 1.2.4.c (v) | Standard treatment plans | 5% | |
(1) In case of disagreement, a
specific procedure should be implemented
(2) The check apply for any entered
data
(3) The check shall attest that the
calculations based on the entered data are correct (see also section
1.2.1)
(4) The case of electronic
densities is given as an example (see related sections)
(5) Minimal set of field sizes :
5x5,10x10,25x25,40x40 (max. square field), 5x10, 5x25,5x40, 10x5,
25x5, 40x5
Repeated checks (see chapter 4)
| Section | Description | Frequency | Tolerance | Extension |
| 4.2.1 | Printing/Plotting device | m | 0.1cm | |
| 4.2.1 | Digitizer | m | 0.1cm | |
| 4.2.1 | Film scanner | m | 0.1cm | |
| 4.2.1 | Computer Tomography - Geometry - Electronic densities |
m y |
0.1cm 0.05/0.1 |
(1) |
| 4.2.1 | Block cutting device | y | no diff | (2) |
| 4.2.1 | Archiving and reading back of patient data see also section 1.2.3.f | y | no diff | |
| 4.2.2 | Monitor Units (MU) - Photon and electron beams |
m |
no diff. |
|
| 4.2.3 | Standard treatment plan - With checksum - Without checksum |
y 3m |
1% 1% |
|
| 4.2.4 | Checksum | m | no diff | (3) |
(1) This check shall also
be executed at each revision of the CT.
(2) This check consist in a
verification of the data transfer.
(3) This check should be included
in the boot procedure of the TPS.
The operator designates the person which performs the routine planning on the TPS
Treatment unit model: All physical and radiation parameters, for a particular piece of medical electrical equipment, that are needed to plan a course of radiotherapy, including dose calculation [13]
Manufacturer could also be mentioned here, but vendor is preferred considering that he is the responsible for the correct functioning of the TPS.
As example, a treatment unit model without the wedge data, or without data concerning some energies available on the actual treatment unit.
The term user shall be understand in the meaning of institution (e.g. the hospital)
Dose per Monitor Unit is a more general term than output factor (see next note).
The British Journal of Radiology [3] defines the output factor as the absorbed dose rate at the point on the beam axis at the depth dm for a given fiel size, normalised to unity for a specified standard field size. Relative output factor can be considered as a synonym for output factor.
This page is maintained by Pierre-Alain Tercier
/ Last updated: 22 September 2011(wws).
Please forward any comments and/or additions to this webpage to:
Webmaster SGSMP.